
2021/11/18
SUMO-ID: a new tool to study the consequences of protein modifications by the ubiquitin family.
Posttranslational modifications (PTM) by the members of the ubiquitin/ubiquitin-like (Ub/UbL) family are essential for cell survival and the proper function of the organism. Cell signalling drives conserved enzymatic pathways to add Ub/UbLs to other proteins, determining the fate of a protein, including changes in its protein-protein interactions, localization or in its stability and degradation. Dysfunction of these modifications are implicated in many illnesses, including cancer, diabetes, neurodegeneration, as well as rare diseases. However, despite their importance, to study Ub/UbL modifications is particularly challenging because they are normally transient and occur in a small percentage of a given protein pool.
How do the interactions of a protein of interest with other proteins change depending on its modifications by members of the ubiquitin family? One ubiquitin-like protein called SUMO is a longstanding research interest to the group of Dr. Rosa Barrio (http://personal.cicbiogune.es/rbarrio/Lab/Welcome.html).
SUMOylation has emerging interest as a therapeutic target for disease, but tools to study its physiological and pathological roles are limited. To study the consequences of PTM by SUMO, the Barrio Group developed SUMO-ID, a new method to reveal the effect of SUMOylation on the molecular interactions for a particular protein, and applicable to other members of the ubiquitin family. SUMO-ID combines the concepts of protein fragment complementation with proximity proteomics and was recently published in the important open-access multidisciplinary journal Nature Communications (https://www.nature.com/articles/s41467-021-26807-6).
SUMO-ID was demonstrated using several proteins of interest. Promyelocytic leukaemia (PML) protein forms PML Nuclear Bodies (PML NBs) and regulates many essential nuclear processes. Applying SUMO-ID to PML, the new work revealed many “writers” (SUMO E3 ligases) and “readers” of PML SUMOylation. These SUMO-dependent interactors of PML participate in transcription, DNA damage, and stress response, and protein SUMOylation itself, revealing new dimensions to the biology of PML NBs. They further applied SUMO-ID to the transcriptional repressor SALL1 and linked its SUMOylation to members of the Nucleosome Remodelling Deacetylase complex (NuRD) and regulators of Wnt signalling.
Finally, they applied SUMO-ID to the tumor suppressor protein TP53, known to be modified by SUMO1 and SUMO2 paralogs, as well as by Ubiquitin. Interestingly, they discovered that each modification favors a different set of preferential interactors, shedding light in the consequences of PTMs of this key regulator of cancer progression.
These new results from the Barrio Group show that SUMO-ID is a specific and sensitive approach that unravels the molecular consequences of Ub/UbL modification and that can be applied to different members of the ubiquitin family. This novel tool will help many investigators explore the role of Ub/UbL modification in their respective research areas and potentially yield new insights and targets for treating disease.
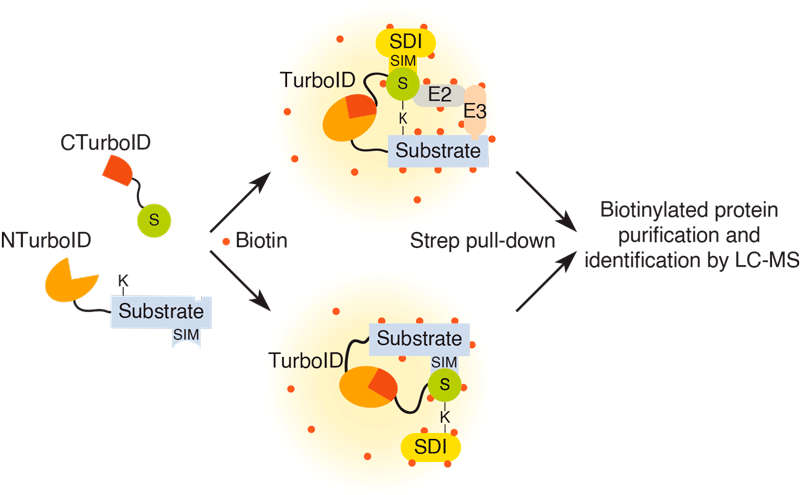
Figure: Schematic representation of the SUMO-ID strategy. CTurboID, C-terminal TurboID; LC-MS: liquid chromatography mass spectrometry; NTurboID, N-terminal TurboID; S, SUMO; SIM: SUMO Interacting Motif; SDI: SUMO-dependent interactor; Strep, streptavidin.

See a large version of the first picture





