2016/05/16
The mechanism of sulfur transfer across protein-protein interfaces: the CSD model system
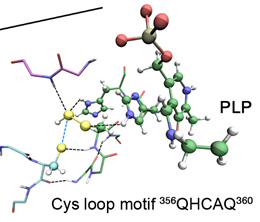
Cellular mobilization of elemental sulfur (S) is a crucial step for the biosynthesis of several entities essential for life, including amino acids, vitamins, and modified RNA nucleosides. However, the exact mechanism behind some of the related transpersulfuration processes remained unknown. Especially, important questions such as how the chemical environment in the active site stabilizes the persulfide on its journey through different protein-protein complexes, why the persulfuration rate may be modulated under different conditions, or how sensitive the chemistry of transpersulfuration is to oxidative stress were still unanswered. In this context, using a multidisciplinary approach combining biophysical, structural biology (NMR and X-ray), and computational methods, a team of scientists from CSIC (CIB & IBMB), the Universities of La Rioja and Grenoble, EMBL, Scripps, CNIO, IRBB, and CIC bioGUNE have unraveled the transfer mechanism between the proteins (CsdA)2 and CsdE, one key model system. These findings have allowed proposing a general mechanism for this vital process.
For more information, see: http://pubs.acs.org/doi/abs/10.1021/acscatal.6b00360
See a large version of the first picture





