2010/10/29
Ubiquitination during neural development
Ubiquitination of neuronal proteins is an essential regulatory mechanism of brain function. The failure of this process has been associated with Parkinson's and Alzheimer's diseases. Ubiquitin proteomic studies performed on neuronal cell lines provided interesting information, but a knowledge gap in the field of in vivo neuronal tissue-specific ubiquitination remains to be filled. Researchers from CIC bioGUNE have been able to identify for the first time the proteins that are ubiquitinated within neurons in vivo under physiological conditions, using the fruit fly model organism.
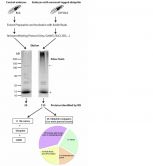
Ugo Mayor's team and collaborators report a novel proteomic strategy based on the in vivo biotinylation of ubiquitin to isolate biotin-ubiquitin conjugates from the neurons of Drosophila melanogaster embryos. Mass Spectrometry data was validated by immunoblotting, to confirm that identified proteins were indeed ubiquitinated in the Drosophila embryonic nervous system (see attached figure). In addition to the ubiquitin substrates, the CIC bioGUNE group has also identified ubiquitin carriers that are active during synaptogenesis. The identification of endogenously ubiquitinated proteins in specific cell types, at specific developmental stages will be crucial for understanding of the tissue-specific functions of essential proteins and their regulation by the ubiquitin system. The strategy described in this work has a tremendous potential to be used in other organisms.
The paper is published in: Molecular and Cellular Proteomics September 22, 2010,
doi: 10.1074/mcp.M110.002188
Manuel S. Rodríguez
See a large version of the first picture





