2013/11/29
Structural basis for the allosteric regulation of human cystathionine beta synthase, the key enzyme of transsulfuration
Cystathionine β-synthase (CBS) is the key enzyme in the transsulfuration pathway controlling the flux of sulfur from methionine to cysteine, which is a precursor of glutathione (the main cellular antioxidant), taurine (the major constituent of bile and essential for cardiovascular function, development and function of skeletal muscle, the retina and the central nervous system) and hydrogen sulfide. Human CBS (hCBS) condenses its two substrates, homocysteine and serine, into cystathionine. Alternatively, it generates cystathionine by condensation of homocysteine and cysteine, or forms lanthionine by condensation of two moles of cysteine. The latter two reactions lead to endogenous production of H2S, a gassotransmitter involved in sulfhydration of proteins, thus in regulation of inflammatory responses and vascular tension. Hereditary CBS-deficiency causes homocystinuria (severely elevated levels of homocysteine), that manifests clinically with connective tissue symptoms such as dislocated lenses and skeletal abnormalities, mental retardation, and thromboembolic episodes. Abnormally elevated CBS activity leads to elevated levels of H2S. These metabolic alterations have been linked with the cognitive disability seen in patients with Down Syndrome and Alzheimer, but also with the development of some type of cancers.
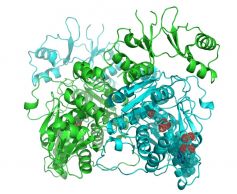
Human CBS associates in dimers or tetramers, and each polypeptide has: (1) an N-terminal heme-binding domain; (2) a central catalytic core containing a PLP molecule, and (3) a C-terminal regulatory domain, which serves as a binding site for the hCBS allosteric activator S-adenosyl-L-methionine (SAM). Binding of SAM to hCBS causes a conformational change with displacement of the C-terminal region, allowing the enzyme to progress from its "inactivated" to its "activated" state.
Until now, the structural information on CBSs was limited to the catalytic core of human CBS (2001) and to the structure of CBS from D. melanogaster (2010). Now, the group headed by Alfonso Martínez de la Cruz, at the Structural Biology Unit of CIC bioGUNE , has taken a big step forward in the field by solving the structure of full-length human CBS in its "inactivated" state", which shows how markedly hCBS differs from insect enzymes. This discovery represents the unique structural basis to understand the SAM-mediated allosteric regulation of human CBS and provides the first 3D map to comprehend the role played by pathogenic mutations located in the regulatory domain and its interface with the catalytic core.
The results have been recently published in the Proc. Natl. Acad. Sci. USA. [Ereño-Orbea J, Majtan T, Oyenarte I, Kraus JP, Martínez-Cruz LA. Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):E3790-9.]
See a large version of the first picture





