2011/03/17
The sirtuin SIRT3 counteracts HIF1A-mediated metabolic reprogramming in cancer
Researchers at Harvard Medical School in collaboration with a group from CIC bioGUNE reveal a tumor suppressive activity for the deacetylase SIRT3 in the regulation of cancer cell metabolism.
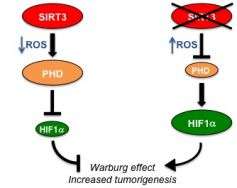
SIRT3 is a NAD-dependent sirtuin that modulates the activity of key mitochondrial enzymes. In this study, SIRT3 is found to oppose the stability of HIF1A through the regulation of its prolyl hydroxylation. In the absence of SIRT3, the increase in reactive oxygen species inhibits prolyl hydroxylation of HIF1A, which in turn promotes its stabilization.
HIF1A, a central regulator of key metabolic enzymes, shifts cellular metabolism towards aerobic glycolisis (catabolism of glucose to lactate, an ATP-inefficient metabolic pathway which is a hallmark of cancer cells), leading to a metabolic state termed the "Warburg Effect". In line with this notion, SIRT3-deficient cells and tumors exhibit increased glucose uptake, lactate production, enhanced glucose-dependent proliferation and exacerbated response to hypoxia. Importantly, SIRT3 is lost at the genomic level in breast cancer specimens, and its reduction correlates with increased HIF1A target expression in tumors. This study uncovers SIRT3 as a potential druggable target in cancer, and highlights the importance of metabolic reprogramming in cancer through the genetic or functional alteration of key metabolic regulators.
Links to the published article:
Article: http://www.cell.com/cancer-cell/abstract/S1535-6108%2811%2900085-7
Pubmed: http://www.ncbi.nlm.nih.gov.gate2.inist.fr/pubmed/21397863
Commentary: http://www.cell.com/cancer-cell/abstract/S1535-6108%2811%2900090-0
See a large version of the first picture





