2012/07/13
HuR plays a critical role in liver fibrosis development
Hepatic fibrosis is the common consequence of chronic liver diseases such as viral and autoimmune hepatitis, alcohol consumption, biliary obstruction, and non-alcoholic fatty liver disease. Activation of hepatic stellate cells (HSC) is the key pathogenic mechanism of fibrogenesis. After continued liver damage, these HSC are exposed to apoptotic hepatocytes, oxidative stress, and inflammatory and profibrogenic factors and undergo a process of activation. These activated HSC increase proliferation and migration, acquire contractility and pro-inflammatory properties to become the major collagen-producing cells. The activation process of HSC is a highly regulated process with hundreds of genes up- and down-regulated.
Human antigen R (HuR) is an RNA-binding protein that plays a major role in control of mRNA turnover and gene expression rates. Both low and high levels of HuR have been correlated with good prognosis in cancer, making careful designs of interventions to modulate HuR functions necessary. These generate the need to study the advantages or disadvantages of HuR silencing in different pathologies, as well as the identification of its specific mediators. During the past years, our group has examined the role of HuR in liver. Our studies have shown that HuR is involved in important functions in the liver, playing a role in hepatocyte proliferation, differentiation and apoptosis, and during hepatocyte malignant transformation. Importantly a study led by Drs. M.L Martinez-Chantar and Marta Varela Rey in the Metabolomics Laboratory 2 at CIC bioGUNE-CIBERehd, has shown that HuR plays a critical role in liver fibrosis development. In the article by Woodhoo A & Iruarrizaga-Lejarreta M and colleagues published in May in Hepatology, the authors have found that HuR silencing has pleiotropic and beneficial functions during liver fibrosis development and HSC activation. They have observed that HuR silencing in a cholestatic liver injury model (Bile Duct Ligation) leads to decreased liver damage, oxidative stress, inflammation, macrophage infiltration and liver fibrosis development. More importantly, the study have shown that HuR regulates HSC activation, which is likely mediated by the effect of HuR in the response of two of the principal mediators of HSC activation, PDGF and TGF-beta. Finally, this work demonstrated that HuR levels in human cirrhotic samples strongly correlate with the degree of HSC activation, suggesting that it could be a valuable therapeutic target for treatment of liver fibrosis, and possibly its progression to hepatocellular carcinoma in humans.
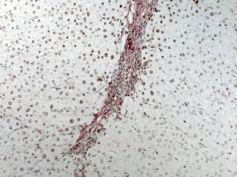
Figure: Immunohistochemistry showing expression of HuR (brown) in activated hepatic stellate cells (Red) in liver sections from cirrhotic patients
See a large version of the first picture





