2020/02/24
CIC bioGUNE researchers identify new molecular mechanisms involved in the regulation of phospholipid biosynthesis in bacteria
(Bilbao, 24 February 2020). Researchers at CIC bioGUNE together with other five scientific groups from Argentina, Brazil and the Basque Country have discovered the molecular mechanism of membrane association of a peripheral enzyme that regulates the phospholipid biosynthesis, an essential pathway for the microbial growth of Gram-positive bacteria. The main results are reported in the Journal of Biological Chemistry and highlighted in the Lipid News Section of ASBMB Today, the member magazine of the American Society for Biochemistry and Molecular Biology.
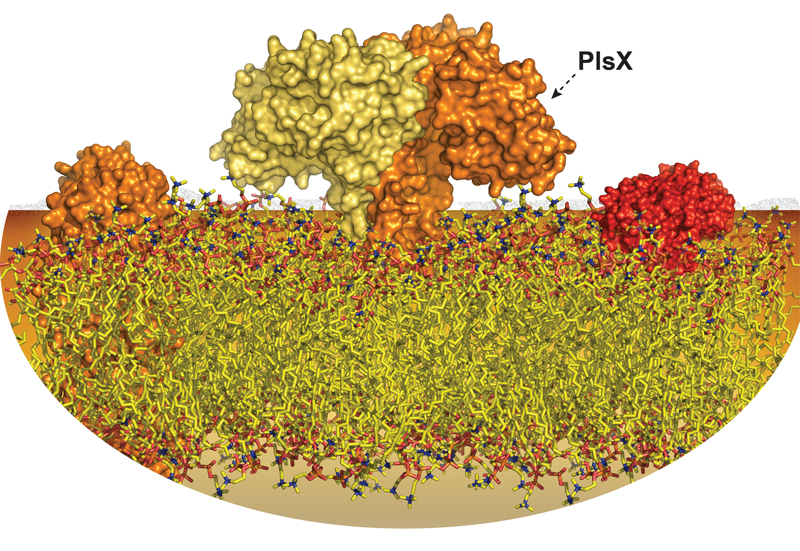
Gram-positive bacteria synthesize phosphatidic acid, the central precursor of membrane phospholipids, using an unusual acyl-phosphate intermediate in a three-step pathway mediated by the phospholipid synthesis enzymes called: PlsX, PlsY and PlsC. Specifically, the first enzyme in this route is the peripheral membrane transacylase PlsX that catalyzes the conversion of acyl-acyl carrier protein to acyl-phosphate and helps coordinate fatty acid and phospholipid biosynthesis. PlsX binds and inserts directly to lipid bilayers, a process mediated by an amphipathic four alpha-helical bundle subdomain that protrudes from the main core of the enzyme.
Based on the protein structure and employing several biophysical approaches, a team lead by Dr. Marcelo Guerin, Ikerbasque Research Professor at CIC bioGUNE, revealed that PlsX membrane binding is mediated by phospholipid charge, whereas unsaturation of fatty acids and membrane fluidity influence membrane insertion. Superficial access to the membrane is not sufficient to ensure efficient delivery of the acyl-phosphate from PlsX to the acyltransferase partner PlsY, which depends on proper and stable insertion of PlsX in the membrane. Such substrate channeling can make this metabolic pathway more efficient and prevents the release of unstable intermediates, protecting them from decomposition and/or diffusion through the aqueous cytoplasm. Therefore, the researchers propose a model in which membrane fluidity governs the membrane insertion of PlsX, which is required for the proper acyl-phosphate delivery to PlsY, thus fluidity might regulate phospholipid biosynthesis in bacteria.
“This model breaks a paradigm in membrane biology, because the membrane fluidity and unsaturated fatty acids, or UFAs, do not seem to be holding hands” explains Dr. Sastre from IFSC-Brazil. At higher membrane fluidity, PlsX inserts deeper into the membrane, but increased UFA content reduces PlsX binding and insertion. Still remains a mystery how can PlsX sense the UFAs, and why do UFAs repell PlsX binding and insertion, however, the investigators suspect that the repulsion effect might be part of a mechanism to downregulate the total phospholipid synthesis observed at low growth temperature.
Peripheral membrane proteins can form cluster as transient oligomers when interacting with lipid bilayers. This crowding would be intensified with increased protein radius and depth of penetration into the hydrophobic region of the membrane. In bacteria, PlsX is mostly homogenously distributed at the bacterial cell membrane, but PlsX foci also are transiently and randomly observed, which could represent physiological oligomeric states related to the regulation of PlsX activity. Interestingly, PlsX inserts deeper into more fluid membranes (containing little or no unsaturated fatty acid content), causing oligomers, including other partners, in the form of foci at the membrane. Consequently, modulating membrane insertion could regulate the substrate channeling as well as the protein crowding, regulating the phospholipid biosynthesis.
These findings highlight the relevance of spatial organization for metabolic pathway functioning and tell us more about how membrane composition and protein mobility can modulate this biosynthesis pathway. “Taking into consideration that this model could be operational for other peripheral enzymes with clinical relevance, and that PlsX/PlsY are presents in pathogenic bacteria and absents in animals, we expect that this contributes for the development of strategies to found new antibacterial agents”, emphasizes Prof. Marcelo Guerin.
See a large version of the first picture





